Radiation risk and safety
Radiation basics
All matter is composed of atoms. Atoms contain a positively charged nucleus made up of protons and neutrons and a negatively charged outer shell made up of electrons. The forces within the atom work to maintain a balance by getting rid of excess energy. In the process, an unstable nucleus may emit a quantity of energy known as radiation.
Over time, radioactive materials "decay" due to the emission of particles from their nuclei. The rate at which this occurs is determined by the radioactive “half-life,” where a half-life is the time taken for half of a radioactive material to decay. For example, starting with 16 radioactive atoms, after one half-life there would be eight atoms. After another half-life there would be four atoms. This process continues until a stable end point is reached. Half-lives can vary from fractions of a second to billions of years depending on the type of material.
Radiation takes the form of electromagnetic waves or high-speed particles. Electromagnetic waves are pure energy with no mass or weight. Examples includes microwaves and X-rays. High-speed particles, on the other hand, have both energy and mass. They include neutrons and alpha and beta particles, such as those used in smoke detectors and some medical therapies. Used nuclear fuel emits both types of radiation.
#DidYouKnow: What is radiation?
Watch now
Ionizing and non-ionizing radiation
Radiation is either “ionizing” or “non-ionizing” depending on how it affects matter. Ionizing radiation has enough energy to create electrically charged particles ("ions") and the potential to break apart molecular bonds or displace electrons from atoms. Non-ionizing radiation, in contrast, leaves behind energy as it passes through matter, but not enough to disrupt molecular bonds. Examples of non-ionizing radiation are heat, light, radio waves and microwaves.
Examples of ionizing radiation are gamma rays, X-rays and alpha particles. Ionizing radiation is emitted from naturally occurring materials (such as uranium, radon and potassium), from man-made materials such as Cobalt-60, used in cancer treatment, and from special equipment such as dental X-ray generators.
Excessive exposure to ionizing radiation can damage living tissue at the molecular level. If the exposure is beyond the body’s natural repair process, it may lead to uncontrolled growth of cells (i.e. cancer) or other serious health effects. Exposure can be controlled through the use of protective shielding barriers.
Exposure limits in Canada
Radiation exposure is measured in "sieverts". A sievert is a unit of radiation "dose" to human tissue that accounts for health effect. Radiation doses are commonly measured in millisieverts (mSv) or thousandths of a sievert.
In Canada, the average effective whole-body dose from natural background radiation is approximately 1.8 mSv per year. The Canadian Nuclear Safety Commission’s radiation dose limit for a member of the public is 1 mSv per year (above and beyond natural background radiation). For nuclear energy workers, the limit is 50 mSv per year, with the total not to exceed 100 mSv during any five-year period.
Studies to date have not been able to prove that exposure below 100 mSv causes cancer or any other health effects. Despite this, a cautious approach to radiation protection is adopted for low doses. It is assumed that all radiation carries some risk and radiation levels should be kept as low as possible.
Radiation and used nuclear fuel
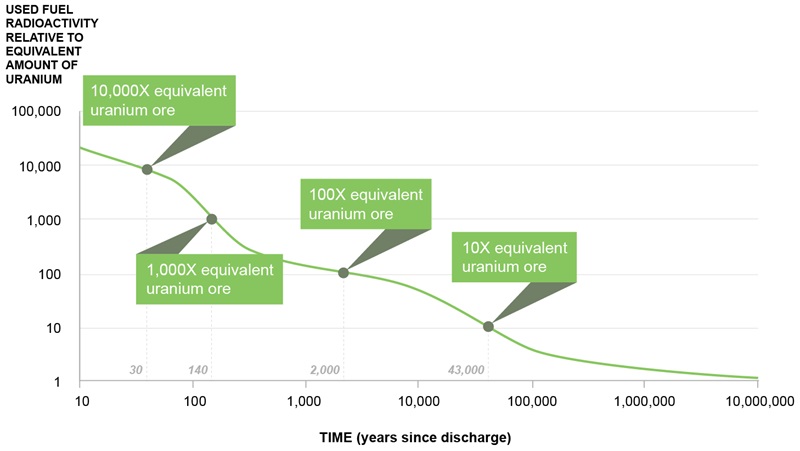
Used nuclear fuel is most radioactive when it is first removed from a reactor. After 10 years of cooling at the reactor site, more than 99 per cent of the radioactivity decays away. While the hazard continues to diminish over time, for practical purposes, used nuclear fuel remains hazardous, essentially indefinitely.
This is a chart depicting the radioactive decay over time of used CANDU fuel. It uses natural uranium as a comparison and shows that it will take up to 10,000,000 years before the used fuel goes back to the same radioactivity levels as natural uranium.
As with the radiological contaminants in the used nuclear fuel, the aim of the repository is to keep these chemically hazardous elements safely contained and isolated. All chemically hazardous elements are addressed in the NWMO’s planning process, and in the safety assessments we conduct to evaluate the long-term safety of a deep geological repository.
Protection from unsafe exposure
In implementing Canada's plan for the long-term management of used nuclear fuel, the NWMO's highest priority is to protect people and the environment from unsafe exposure to radiation. This responsibility includes both the transportation of used nuclear fuel and its containment and isolation in a deep geological repository. In the surface facilities at the repository, used nuclear fuel will be shielded at all times to ensure radiation doses to members of the public and operating staff are as low as reasonably achievable and well below regulatory limits. In the deep geological repository, a series of engineered and natural barriers will work together to contain and isolate used nuclear fuel.
Learn more about the NWMO's commitment to safety and security, as well as regulatory oversight of used nuclear fuel in Canada.